Supercritical CO2 Extraction (SCFE) is the process of separating one component (the extractant) from another (the matrix) using CO2 as supercritical fluids as the extracting solvent.
The process can be described with three keywords: Extraction, Separation and Condensation.
First, liquid CO2 is super-cooled and fed into the
pump. Due to heavy compression, the CO2 is heated up and transferred into a
supercritical state. Under these conditions, it possesses the properties of a
gas and a liquid and is characterised primarily by its excellent dissolving
capacities.
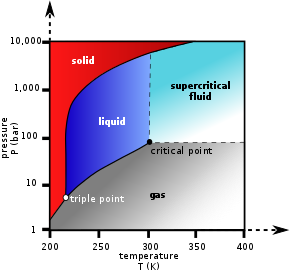
Extraction conditions for supercritical carbon dioxide
are above the critical temperature of 31°C and critical pressure of 74bar.
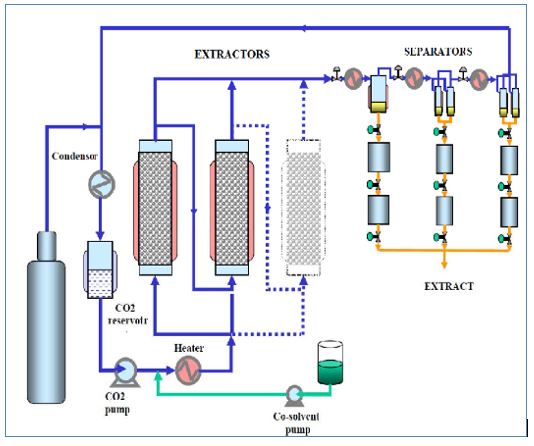
The high fluidity of supercritical CO2 allows it to
penetrate into the smallest pores of target Raw material and extract the
desired substances from the raw material. The supercritical CO2 is then
expanded and heated up. It turns back into a gas and evaporates without a
trace, leaving nothing but the pure extract.
As CO2 is inert, it neither reacts with the extract
nor distorts its properties.
